TMS
Transcranial
Magnetic Stimulation
FDA-Cleared TMS Therapy
TMS therapy is a non-invasive treatment in which a strong electromagnetic field generated by a coil penetrates the skull and stimulates nerve cells. The electromagnetic field reaching the cerebral cortex induces electric currents within neurons, activating or inhibiting brain activity. To date, the U.S. FDA has cleared TMS for the treatment of major depressive disorder, anxiety, obsessive-compulsive disorder, and smoking addiction. It is also being studied as a solution for various drug-resistant and refractory conditions. TMS is often referred to as an “electroceutical” that treats diseases without medication and was selected as one of the World Economic Forum’s Top 10 Emerging Technologies in 2018.
Reborn With REMED TMS Therapy
TMS therapy is a treatment solution performed without anesthesia, incision, pain, or significant side effects.
Based on the principle of modulating the activity of brain neurons, TMS technology applies customized protocols for each condition and delivers patient-specific stimulation locations and intensities. It provides an effective treatment solution not only for psychiatric disorders such as depression and anxiety, but also for refractory conditions including stroke, autism, dementia, Parkinson’s disease, developmental disorders, and tinnitus.
TMS Therapy Is Recommended For
Treatment outcomes may vary depending on the indication and individual patient condition.
TMS
A Treatment Solution Without Anesthesia or Incision
TMS Accessories
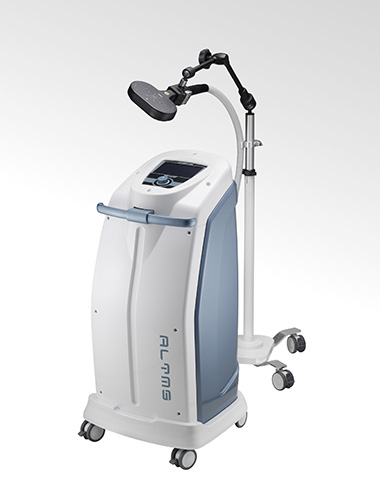
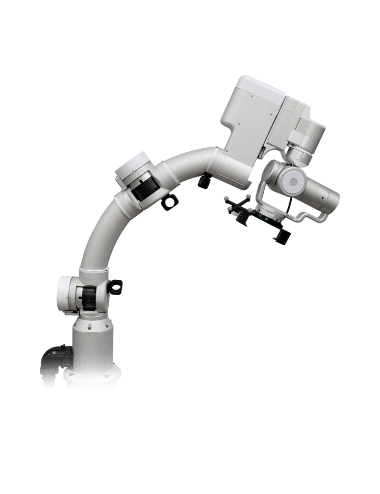
ALTMS
- First FDA-Cleared TMS System in Asia Exported to Over 30 Countries Worldwide
- REMED’s ALTMS has obtained U.S. FDA clearance and European CE certification. It is currently used in more than 500 major hospitals and clinics in Korea (approximately 90% domestic market share) and is the only Korean TMS device exported to over 30 countries, including the United States, Japan, and Europe.
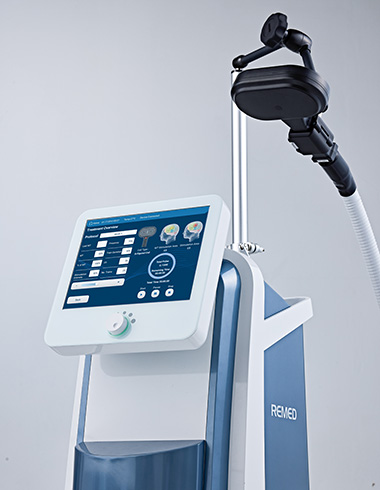
BrainStim
- Next-Generation High-Performance TMS Hybrid Cooling System Combining Liquid and Air
- BrainStim is an advanced TMS system that provides over 40 disease-specific protocols and includes a personalized data save program, enabling intensive and customized patient care.
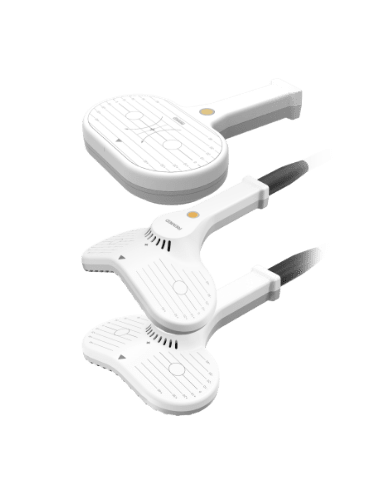
- TMS COILS
- Provides three types of coils optimized for specific TMS treatment targets.
Each coil is categorized by indication and designed to deliver deeper stimulation.
-

Traditional 8-figured Coil
-

Double Cone Coil 120º
-

Angulated 8-figured Coil 150º
BrainStim 100
- Portable TMS Effective Treatment, Easy to Use, Compact in Size
- REMED’s helmet-type TMS system, BrainStim 100, is designed to lead the next era of TMS therapy. It removes spatial and time limitations associated with conventional in-clinic TMS treatment and provides a more convenient treatment environment. With safe output and adjustable intensity, BrainStim 100 enables comfortable and simple therapy in the patient’s preferred setting.